
CBS
|
Call us now! 651.964.6461 or 877.601.STOP (7867)
ABSOLUTELY WORKS FOR PEOPLE ON BLOOD THINNERS

Full study is available upon request
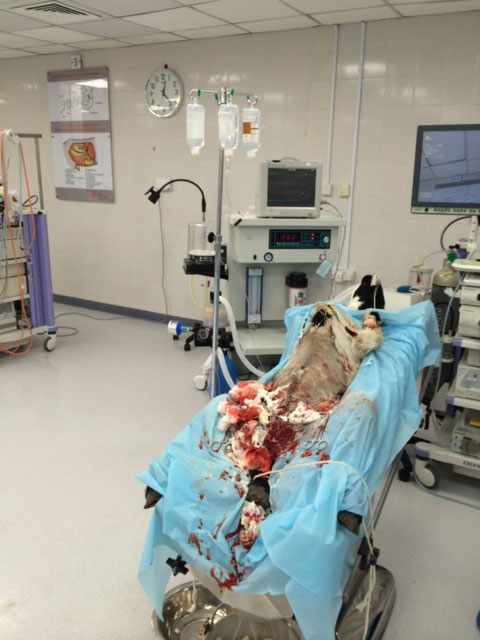
This animal study, conducted by five independent medical doctors, was
performed on swine by completely severing femoral arteries. The
results were peer reviewed, it was presented at a major medical
conference, and it has been published in a major medical journal.
The FDA has not granted StopsBleeding™ a claim for arterial bleeding. However, this study indicates its effectiveness on swine. StopsBleeding™ has been approved for moderate to severe bleeding wounds for humans by the FDA with an Rx prescription, and the FDA has allowed the claim of "Safe in the Wound" for humans. The FDA has also granted StopsBleeding™ a claim for percutaneous arterial access sites.

The FDA has not granted StopsBleeding™ a claim for arterial bleeding. However, this study indicates its effectiveness on swine. StopsBleeding™ has been approved for moderate to severe bleeding wounds for humans by the FDA with an Rx prescription, and the FDA has allowed the claim of "Safe in the Wound" for humans. The FDA has also granted StopsBleeding™ a claim for percutaneous arterial access sites.


Also Tested by the United Arab Emirates Zayed Military Base and the Egyptian Military • Utilized by the largest trauma center in Iraq
FDA Cleared
"Safe in the Wound"
NAMSA GLP Independent Laboratory Animal Study
(Full Study is Available)

Conclusion:
The objective of this study was to evaluate local tissue response to StopsBleeding™ hemostatic powder in a chronic rabbit subcutaneous implant model. There were no adverse events, clinical observations, abnormal clinical pathology, or abnormal gross pathology attributed to the test article.
To paraphrase the study, all animals observed after the 4-week period were in the same condition as when they started. The StopsBleeding™ powder in the animals' bodies had no adverse effect on any of the animals or in any of the sites.
Summary:
The in-life assessment for each animal was clinically normal upon entering the study and all animals remained healthy during the in-life portion of the study. There were no adverse events noted during the study. According to the physical examinations, the clinical pathology results, the daily observations, the procedures, and the in-life assessment all animals in this study demonstrated adequate health to assess the objective of the study.
The objective of this study was to evaluate local tissue response to StopsBleeding™ hemostatic powder in a chronic rabbit subcutaneous implant model. There were no adverse events, clinical observations, abnormal clinical pathology, or abnormal gross pathology attributed to the test article.
To paraphrase the study, all animals observed after the 4-week period were in the same condition as when they started. The StopsBleeding™ powder in the animals' bodies had no adverse effect on any of the animals or in any of the sites.
Summary:
The in-life assessment for each animal was clinically normal upon entering the study and all animals remained healthy during the in-life portion of the study. There were no adverse events noted during the study. According to the physical examinations, the clinical pathology results, the daily observations, the procedures, and the in-life assessment all animals in this study demonstrated adequate health to assess the objective of the study.
StopsBleeding™ is approved in the United Arab Emirates, India, the Middle East, Mexico, many parts of Africa, and the United States.